PHASE I REPORT (December 2000) |
|
|
|
|
| Table mountain pine | 20.0 | 2 |
| Sweetgum | 5.6 | 3 |
| Sycamore | 31.2 | 89 |
| Winged sumac | 3.3 | 5 |
| Black cherry | 11.5 | 10 |
| Tall milkweed | 0.3 | 0 |
| Black-eyed Susan | 12.8 | 50 |
| Dwarf dandelion | 0.3 | 0 |
| Yellow buckeye | 4.7 | 3 |
| Virginia pine | 30.0 | 50 |
| Cutleaf coneflower | 5.5 | 3 |
Table O-2. W126 (ppm-h) and N100 (number of hourly average concentrations greater than or equal to 0.1 ppm) exposure levels that resulted in a 10 percent growth loss for selected plant species (from Lefohn 1998.)
Name
W126 (ppm-h)
N100
Aspen 259
6.4
4
Aspen wild
71.4
243
Black cherry
6.5
1
Red maple
85.4
245
Whorled-wood aster
8.2
10
Yellow poplar
14.4
4
Eastern white pine
30.2
66
Sugar maple
44.7
131
Sycamore
15.4
27
Winged sumac
9.7
4
|
|
|
|
| Aspen 259 | 6.4 | 4 |
| Aspen wild | 71.4 | 243 |
| Black cherry | 6.5 | 1 |
| Red maple | 85.4 | 245 |
| Whorled-wood aster | 8.2 | 10 |
| Yellow poplar | 14.4 | 4 |
| Eastern white pine | 30.2 | 66 |
| Sugar maple | 44.7 | 131 |
| Sycamore | 15.4 | 27 |
| Winged sumac | 9.7 | 4 |
Ambient W126 and N100 values are available for many Class I areas in the eastern U.S., with values soon to be available for additional FLM areas. A table showing representative high and low W126 and N100 values for selected FLM areas is appended to this report (Appendix 3.B). Unfortunately, ambient ozone data are lacking for many western U.S. FLM areas, and large differences in terrain and elevation may limit the use of nearby data.
d. Identification of Ozone Sensitive AQRVs or Sensitive Receptors
FLMs have determined that given the high ecological, aesthetic, and intrinsic value of federal lands, all native species are significant and warrant protection. Ideally, protection efforts would focus on the identification and protection of the most sensitive species in an area. Unfortunately, AQRV identification is limited by incomplete species inventories and/or lack of exposure/response data for most species of native vegetation. Sensitive species identification will improve as more information becomes available. In the meantime, FLAG is providing a preliminary list of sensitive plant species for each Class I area, i.e., those species that have been observed to exhibit ozone symptoms at ambient ozone exposures (Appendix 3.A). Those ambient levels have not necessarily occurred at the specific Class I area where the plants occur. AQRV lists will be available in the Air Synthesis and NRIS-AIR databases (See Section B.4.f. of this report) and will be updated as necessary.
e. Review Process for Sources that Could Affect Ozone Levels
or Vegetation in FLM Areas
As mentioned above, NOx and
VOC are ozone precursors. States and the EPA have based ozone
control strategies in various parts of the country on the determination
of which precursor is most likely to influence the formation
of ozone. Information suggests that in areas where ozone formation
is driven by VOC emissions, i.e., VOC-limited areas, VOC to NOx
ratios are less than 4:1. In VOC-limited areas, minimizing or
reducing VOC emissions is the most effective means of limiting
or lowering ozone concentrations. Conversely, in NOx-limited
areas, where VOC to NOx ratios are greater than 15:1, controlling
NOx emissions is most effective. It is generally thought that
most rural areas of the U.S. are NOx-limited, most or all of
the time, with the possible exception of the rural areas of southern
California. The FLMs do not have current data to show that all
areas are NOx limited, nor do they consider VOCs to be unimportant
as ozone precursors. However, until there is enough information
available for FLAG to determine whether ozone formation in each
FLM area is primarily limited by NOx or VOC emissions, we will
assume all FLM areas are NOx-limited and will focus on control
of NOx emissions. Where FLMs have information indicating a specific
area is VOC limited, they will shift ozone protection strategy
to focus on VOC rather than NOx emissions.
Source/receptor modeling is required in most NSR permit applications
for particulate matter, sulfur dioxide, and nitrogen dioxide.
FLAG is aware of attempts by EPA and others to develop dispersion
models that can relate emissions from a single source to changes
in ozone concentrations. We recognize that there is currently
no model available that can provide this kind of single source
attribution information for ozone. Nevertheless, because of existing
and suspected ozone concerns in a number of FLM areas (e.g.,
evidence of phytotoxic effects and high ambient concentrations),
we will consider ozone effects when reviewing NSR permit applications.
However, because single source attribution modeling is possible
for both visibility and deposition, FLMs will be more concerned
about ozone if modeling indicates NOx emissions are likely to
cause an adverse impact on visibility, soils, and/or surface
waters.
The FLMs recognize that oxidant stipple can occur at hourly ozone concentrations that can be considered natural background levels (Singh et al. 1978). Many of the high hourly background concentrations can be attributed to stratospheric intrusions or stratospheric mixing in the upper troposphere (Singh et al. 1978); but stratospheric intrusions rarely occur in the middle and southern latitudes after May (Singh et al. 1980, Wooldridge et al. 1997), and thus do not coincide with the major portion of the growing season. However, oxidant stipple has been observed on foliage in the spring when these intrusions can occur. In general, oxidant stipple observed on foliage from June through September cannot be attributed to natural background ozone from stratospheric sources. Low levels of ambient ozone may occasionally occur in the troposphere from non-anthropogenic and non-stratospheric sources.
The occurrence of oxidant stipple necrosis on plant foliage may indicate further ozone induced physiological and growth impacts. Point sources emit precursors that could produce ozone at the FLM area, and increased ozone could induce further injury or damage to vegetation. However, we assume that restriction on increases in ozone precursors will prevent additional ambient ozone and subsequent increases in injury or damage to vegetation in FLM managed areas. It is important that ambient ozone monitoring be conducted by the State or Local air pollution control agency or by the FLM to determine the seasonal ozone exposure.
FLM actions or specific requests on a permit application will be based on the existing air pollution situation at the FLM area(s) that may be affected by the source. Some FLMs may rely on growth loss rather than foliar necrosis to make an adverse impact. Each FLM will determine if actions are warranted to limit emissions that might lead to increased ambient ozone, based on the expected impact of ozone in their particular area.
FLM response will depend on whether or not:
1. ozone vegetation effects have been documented in the area (as evidenced by foliar injury or damage to vegetation);
2. ozone exposure levels occurring in the area are high enough that they could affect vegetation (i.e., ozone exposures are at levels shown to be phytotoxic).
Figure O-1 outlines the general FLM process for responding to NSR permit applications based on ozone exposure and vegetation effects at the receptor site. Management decisions regarding acceptance of an existing or future ozone exposure will be area-specific and may differ significantly between agencies, or even regionally within agencies. Each FLM will determine if injury and/or damage are necessary to warrant action, based on the expected impact in the area they manage. The decisions are based on the FLM interpretation of regulations, past experience in the NSR arena, availability of ozone effect exposure/response information for species that occur in the area, and other factors. The FLM will negotiate with the NSR permit applicant and the permitting authority regarding the options listed in Figure O-1.
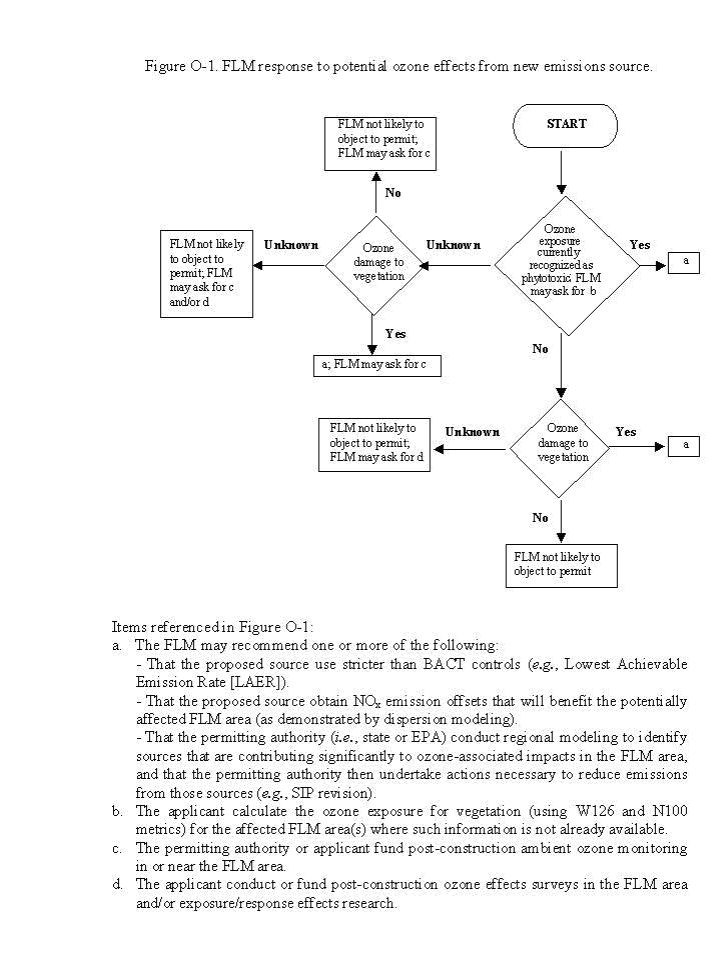
Note: "Ozone exposure currently recognized as "phytotoxic" is determined based on data from exposure response studies and ambient ozone at the site. The FLM may ask the applicant to calculate the ozone exposure values if these data are not already available. "Ozone damage to vegetation" is determined from field observations at the impacted site."
f. Further Guidance to FLMs
As mentioned above, limited information about ozone exposure/response relationships in plants and lack of an ozone source/receptor model make it difficult to protect FLM areas from the effects of ozone from new sources. However, there are other area-specific gaps in information that also limit protection efforts. It is important for local land managers to attempt to collect the missing information. This section provides guidance specifically to FLMs on what types of data should be collected and how the data could be collected.
Identifying and Monitoring
Ozone-sensitive AQRVs
Many FLM areas need more details regarding plant species presence,
location, and abundance. FLAG recommends FLMs gather this information,
where needed, and refine their lists of area-specific ozone-sensitive
plants. FLMs are currently developing lists of sensitive species
to crosscheck with the plant species list for their area to determine
potential sensitivity to ozone. In the future, the FLMs will
place ozone sensitive plant species lists in the NRIS-AIR or
Air Synthesis databases for comparison to plant species lists
for each wilderness area or national park.
FLAG recommends that once local FLMs have developed lists of
potentially sensitive AQRVs specific for their site, they conduct
surveys to detect the presence of ozone-induced foliar injury
on the selected species. The USDA/FS Forest Health Monitoring
(FHM) Program has developed foliar injury survey protocols and
QA/QC procedures that can be used to collect this information.
Another resource is the foliar injury training module developed
by the NPS Air Resources Division and Pennsylvania State University.
This module helps field staff identify and quantify ozone injury
symptoms on plant foliage. Field crews must obtain proper training
and field experience in identifying foliar injury symptoms before
surveys can be conducted.
Ideally, to verify ozone-induced foliar injury symptoms in the field, exposure/response fumigation studies should be conducted on these species, using concentrations that reflect current ambient exposure. Plants should also be tested at higher exposures, simulating increased levels of ambient ozone that might occur in the future. Due to the expense of constructing and operating such systems, it would be most appropriate for agencies to join resources and develop regional fumigation facilities. At a minimum, such facilities should be constructed both in the eastern and western U.S., since ambient conditions at an eastern facility might not be appropriate for western species and vice versa.
Ambient Ozone Monitoring
Many FLM areas do not currently have either on-site or nearby ambient ozone monitoring data. FLAG recommends that local FLMs make every effort to collect this information and that they use quality-assured ambient ozone monitoring protocols developed by the EPA and the state air quality agency. Continuous (active) monitoring is preferred since this type of data is necessary to determine compliance with the ozone NAAQS. Continuous monitoring is also necessary to determine the temporal dynamics of ozone exposure for vegetation, and is necessary to calculate the W126 and N100 parameters. Unfortunately, continuous monitoring is expensive and requires electric power that is often not available in or near remote FLM areas. When installing a continuous monitor is not an option, FLAG recommends use of passive monitors. Passive monitors give total exposure loading values (SUM00) for a specified period of time. The data are useful for indicating year-to-year changes in total ozone exposure at an individual site, and for indicating where continuous monitors should be installed. However, FLMs recognize the limitation of passive samplers in relating ozone exposure to plant response.
g. Ozone Air Pollution Web Sites
U.S. EPA ozone information:
http://www.epa.gov/airlinks
http://www.epa.gov/oar/oaqps/cleanair.html
http://www.epa.gov/ttn/oarpg/naaqsfin/o3health.html
http://www.epa.gov/castnet
NPS ozone information:
http://www.nature.nps.gov/air/Monitoring/network.cfm
http://www.nature.nps.gov/air/Pubs/bioindicators/index.cfm
Ozone effects research, USDA
ARS, North Carolina:
http://www.ces.ncsu.edu/depts/pp/notes/Ozone/ozone.html
Ozone effects research, England:
http://www.ncl.ac.uk/airweb/ozone/ozone.htm
Ozone effects research, Switzerland:
http://www.wsl.ch/forest/risks/
Ozone exposure metrics for vegetation:
http://www.asl-associates.com/
Appendices
To view the complete set of Appendices, please visit:
http://www.nature.nps.gov/air/Permits/flag/flagDoc/subGroup3.cfm
H. Bibliography
Ashmore, M.R. and A.W. Davison. 1996. Towards
a critical level of ozone for natural vegetation. In: Kaerenlampi,
L. and L. Skaerby, eds. Critical Levels for Ozone in Europe:
Testing and Finalizing the Concepts. UN-ECE Workshop Report.
University of Kuopio, Department of Ecology and Environmental
Science. p. 58-71.
Brace, S., D.L. Peterson and D. Horner. 1998. Diagnosing Ozone
Injury in Vascular Plants of the Pacific Northwest. PNW-GTR-xxx
(In Press). U.S. Department of Agriculture, Forest Service, Pacific
Northwest Research Station.
Chappelka, A.H., L.J. Samuelson, J.M. Skelly, A.S. Lefohn and
D. Nesdill. 1995. Effects of Ozone on Vegetation in Southern
Appalachians: an Annotated Bibliography. Auburn University, School
of Forestry. Auburn, Alabama.
Davis, D.D. 1995. Evaluation of Ambient Ozone Injury on the Foliage
of Vegetation in the Edwin B. Forsythe National Wildlife Refuge,
Brigantine, New Jersey. Final Report.
Davis, D.D. 1998. Evaluation of Ambient Ozone Injury on the Foliage
of Vegetation in the Cape Romain National Wildlife Refuge, South
Carolina. 1997 Observations.
Davis, D.D. 1998. Evaluation of Ozone Injury on Vegetation in
the Okefenokee National Wildlife Refuge, Georgia. 1997 Observations.
Eckert, R., R. Kohut, J. Laurence, P. King, T. Lee, B. Rock,
D. Moss and A. Theisen. 1991. Studies to Assess the Effects of
Ozone on Native Vegetation of Acadia National Park. Annual Report.
University of New Hampshire. Durham, New Hampshire.
Guderian, R., D.T. Tingey, and R. Rabe. 1985. Effects of photochemical
oxidants on plants. Part 2. In: Guderian, R., ed. Air Pollution
by Photochemical Oxidants. Springer-Verlag. New York. p. 129-296.
Guderian, R. 1977. Air Pollution. Phytotoxicity of Acidic Gases
and Its Significance in Air Pollution Control. Springer-Verlag.
New York.
Heck, W.W. and E.B. Cowling. 1997. The need for a long-term cumulative
secondary ozone standard - an ecological perspective. Environmental
Management. January.
Heck, W.W., C.S. Furiness, C.K. Simms and E.B. Cowling, eds.
1997. Report from Workshop on Research Needs to Assess the Effects
of Ozone on Crop, Forest and Natural Ecosystems. May 18, 1997.
Southern Oxidants Study, North Carolina State University. Raleigh,
North Carolina.
Kaerenlampi, L. and L. Skaerby, eds. 1996. Critical Levels for
Ozone in Europe: Testing and Finalizing the Concepts. UN-ECE
Workshop Report. University of Kuopio, Department of Ecology
and Environmental Science.
Lefohn, A.S. 1998. The Identification of Ozone Exposures that
Result in Vegetation Visible Injury and Growth Loss for Specific
Species Grown in the Southern Appalachian Mountain Region. Southern
Appalachian Mountains Initiative Report.
Lefohn, A.S. and V.C. Runeckles. 1987. Establishing a standard
to protect vegetation - ozone exposure/dose considerations. Atmospheric
Environment 21(3):561-568.
Massman, W.J., R.C. Musselman, and A.S. Lefohn. 2000. A conceptual
ozone dose-response model to develop a standard to protect vegetation.
Atmospheric Environment 34:745-759.
Miller, P., R. Guthrey and S. Schilling. 1995 Draft. Third Progress
Report of FOREST. Comparisons of 1991-1995 Ozone Injury Index
at Sierra Nevada and San Bernardino Mountains Sites. 9 pp.
Miller, P.R., K.W. Stolte, D.M. Duriscoe and J. Pronos. 1996.
Evaluating Ozone Air Pollution Effects on Pines in the Western
United States. General Technical Report PSW-GTR-155. U.S. Department
of Agriculture, Forest Service, Pacific Southwest Research Station.
Albany, California.
Miller, P., R. Guthrey, S. Schilling and J. Carroll. 1996. Ozone
injury responses of ponderosa and Jeffrey pine in the Sierra
Nevada and San Bernardino Mountains in California. Paper presented
at International Symposium: Air Pollution and Climate Change
Effects on Forest Ecosystems, Feb 5-9, 1996. U.S. Department
of Agriculture, Forest Service, Pacific Southwest Research Station.
Riverside, California.
Multi-stakeholder NOx/VOC Science Program. 1997. Report of the
Vegetation Objective Working Group: Canadian 1996 NOx/VOC Science
Assessment. Atmospheric Environment Service, Environment Canada.
Toronto, Ontario, Canada.
Musselman, R.C. and T.J. Minnick. 2000. Nocturnal stomatal conductance
and ambient air quality standards for ozone. Atmospheric Environment
34:719-733.
Neufeld, H.S. and J.R. Renfro. 1993a. Sensitivity of Black Cherry
Seedlings (Prunus serotina Ehrh.) to Ozone in Great Smoky Mountains
National Park. The 1989 Seedling Set. Natural Resources Report
NPS/NRTR-93/112. U.S. Department of the Interior, National Park
Service, Air Quality Division. Denver, Colorado. 26 pp.
Neufeld, H.S. and J.R. Renfro. 1993b. Sensitivity of Sycamore
Seedlings (Platanus occidentalis) to Ozone in Great Smoky Mountains
National Park. Data from 1989. Natural Resources Report NPS/NRTR-93/131.
U.S. Department of the Interior, National Park Service, Air Quality
Division. 39 pp.
Singh, H.B.; Ludwig, F.L.; Johnson, W.B. 1978. Tropospheric ozone:
concentrations and variabilities in clean remote atmospheres.
Atmospheric Environment 12:2185-2196.
Singh, H.B.; Viezee, W.; Johnson, W.B.; Ludwig, F.L. 1980. The
impact of stratospheric ozone on trpospheric air quality. Journal
Air Pollution Control Association 30:1009-1017.
Skaerby, L. and P.E. Karlsson. 1996. Critical levels for ozone
to protect forest trees - best available knowledge from the nordic
countries and the rest of Europe. In: Kaerenlampi, L. and L.
Skaerby, eds. Critical Levels for Ozone in Europe: Testing and
Finalizing the Concepts. UN-ECE Workshop Report. University of
Kuopio, Department of Ecology and Environmental Science. p. 72-85.
U.S. EPA. 1992. Summary of Selected New Information on Effects
of Ozone on Health and Vegetation: Supplement to 1986 Air Quality
Criteria for Ozone and Other Photochemical Oxidants. EPA/600/8-88/105F.
Research Triangle Park, NC: US Environmental Protection Agency,
Office of Health and Environmental Assessment.
U.S. EPA. 1996a. Review of National Ambient Air Quality Standards
for Ozone Assessment of Scientific and Technical Information.
OAQPS Staff Paper. EPA-452/R-96-007. U.S. EPA, Office of Air
Quality Planning and Standards. Research Triangle Park, North
Carolina.
U.S. EPA. 1996b. Air Quality Criteria for Ozone and Related Photochemical
Oxidants. Vol. 2. EPA/600/P-93/004bF. U.S. EPA, Office of Research
and Development. Research Triangle Park, North Carolina.
Wooldridge, G; Zeller, K.; Musselman, R. 1997. Ozone concentration
characteristics at a high-elevation forest site. Theoretical
and Applied Climatology 56:153-164.
Copyright © 1995-2026 A.S.L. & Associates. All rights reserved.
Privacy Notice